Reference_Readme
The so called "anti z-cosy" is an experiment which has purely absorptive and in-phase lines along the diagonal. The cross peaks are absorptive as well but do
have anti phase splittings. This 2D gives COSY spectra with simplified and sharp peaks for tracing correlations. However, it also can be sheared to give
chemical shifts along one dimension and J-couplings along another. This can be quite useful for measuring J-couplings in a straightforward manner without
the usual guess work. Lastly, the 'sheared' spectra can be further utilized to extract a 1D proton NMR which is free of J-coupling. That is, it looks much like
a decoupled carbon spectrum. Consider the anti z-cosy spectrum of ethyl cinnamate and zoom in on the aromatic and ethylene peaks (A/B).
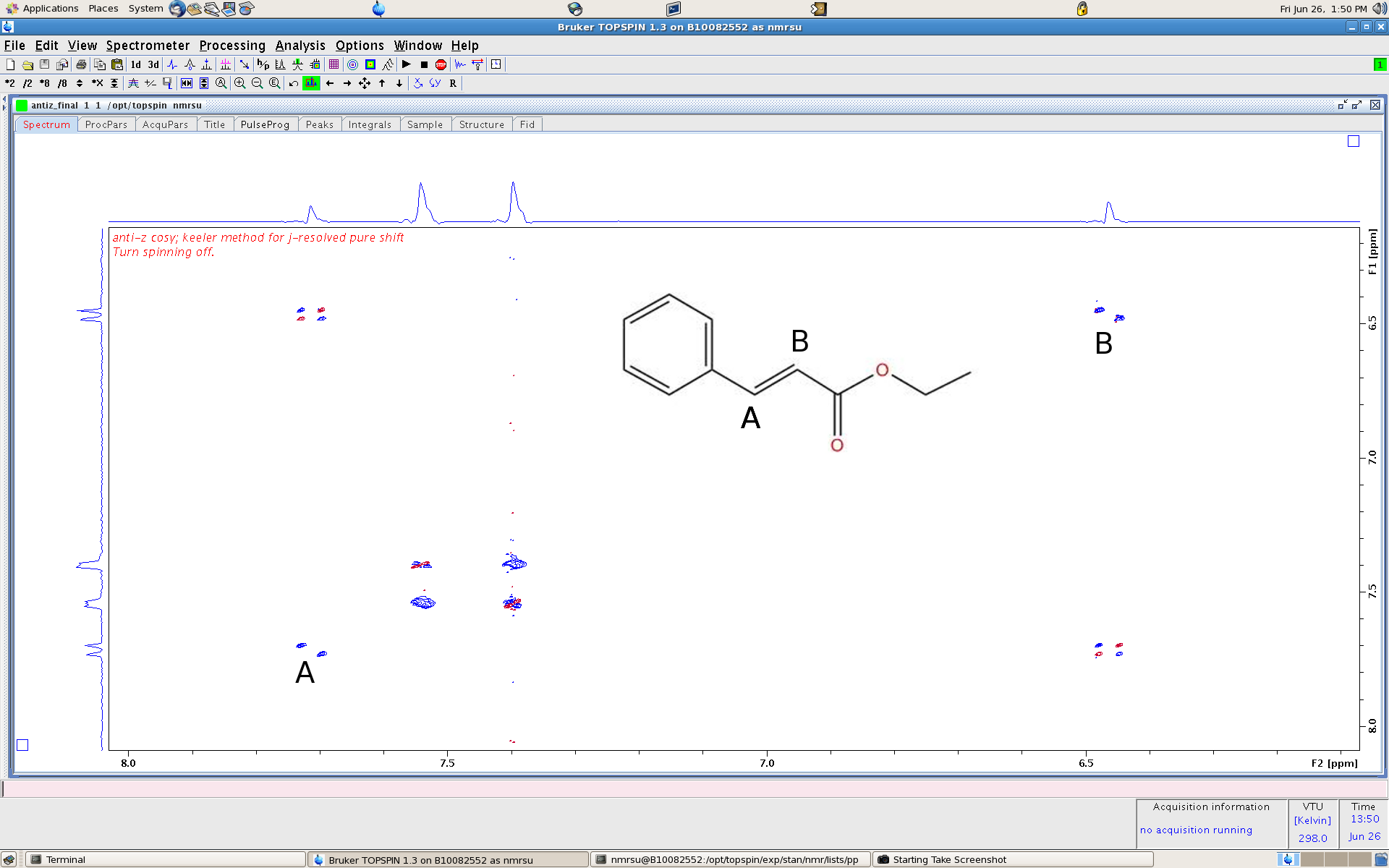
Note the peaks along the diagonal show the expected doublets for the ethylene group and are tilted 45 degrees. we usually would see a doublet of doublets here..
the cross peaks still show the familiar splitting but now the peaks are phase sensitive as well (sharp). use this spectrum for a decent cosy spectrum if you like.
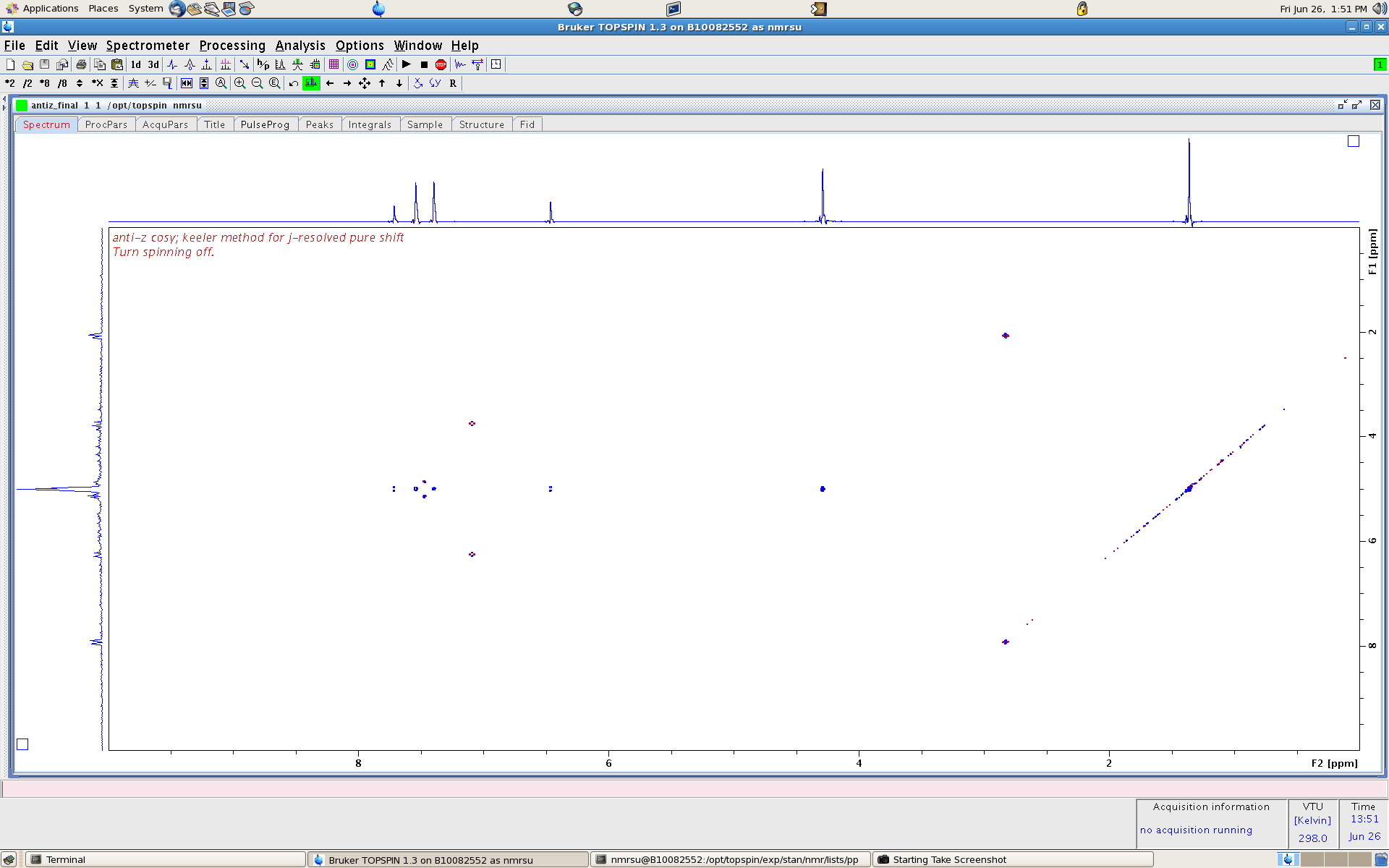
now imagine tilting the entire spectrum 45 degrees. what we would get is a horizontal with chemical shift only in one dimension and in vertical the corresponding
J-coupling. This is called a 2D J-Resolved spectrum, please see below:
Lets zoom in closer to the CH2 in the ethyl group and horizontal slice right down the middle of the spectrum:
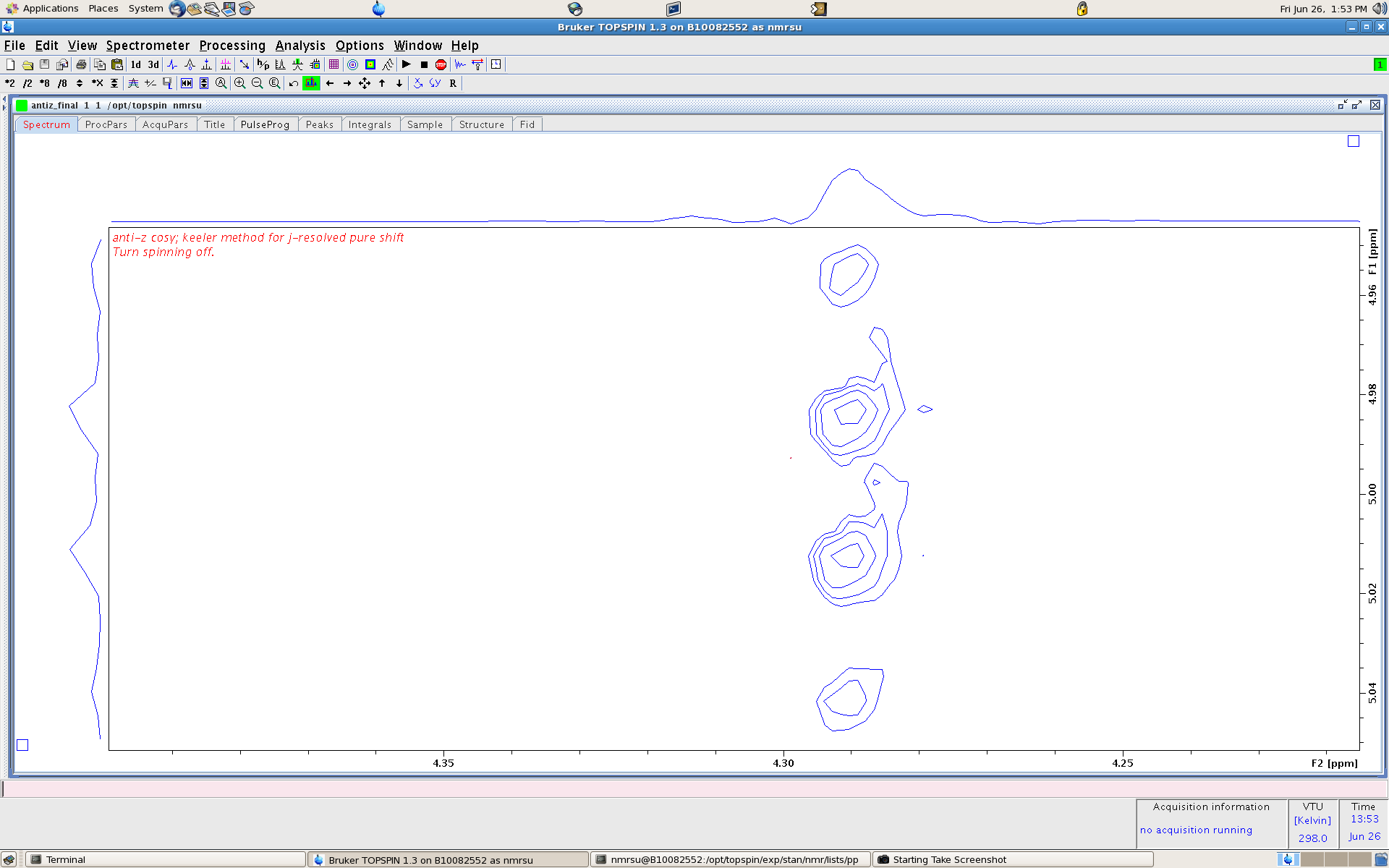
Now the J-couplings can be read directly along the vertical axis (F1 dimension) of the 2D. It turns out that if you add up all the horizontal slices for
the splittings along the F1 dimension (as seen above) then a 1D free of J-coupling can be reconstructed which largely integrates correctly for the number of protons as
well (more on this later).
be sure to lock, shim and tune proton before starting the data acquisition
- rpar azcosy.bbo all - read in the parameter file anti z-cosy
- ns - number of scans set to 2 right now (expt takes 2 hours). increase if s/n is poor
- rga - sets the receiver gain (rg) which depends on the sample concentration (wait)
- zg - zero memory buffer and go
- xfb - 2D Fourier transform and line broadening
- you should see the 2D COSY now with the diagonals all in phase
- shearj - this is a shearing AU program provided by the Keeler group. It tilts the spectrum 45 degrees (takes 5s)
- zoom into the horizontal region which has all the peaks pointing up. ignore the red peaks as these are cross peaks and not useful here.
- It is useful to cl H/P icon and change the vertical axis to units of Hertz. Now it is easy to measure the J-couplings directly.
- note that the j-couplings here are 2X's too large so divide by 2 to get actual value. i think there is a bug in their shearing routine
- proj - this launches a screen to extract defined slices and add them into a 1D
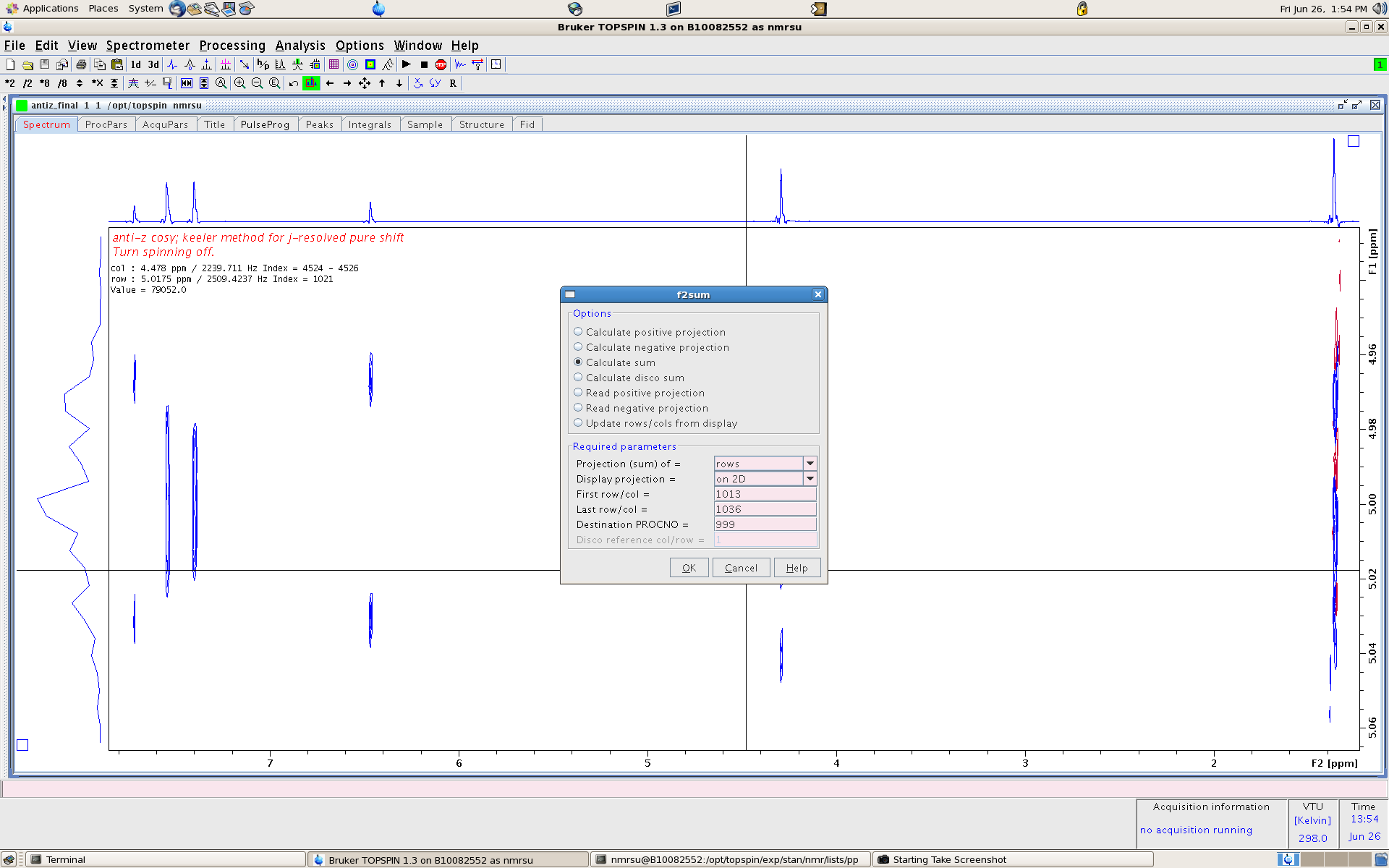
- Note here we use the cursor to determine the index values to plug in. here it is 1013 and 1036. also note the 1D is saved in proc 999
- re 1 999 - this reads in the 1D expt 1 and process 999
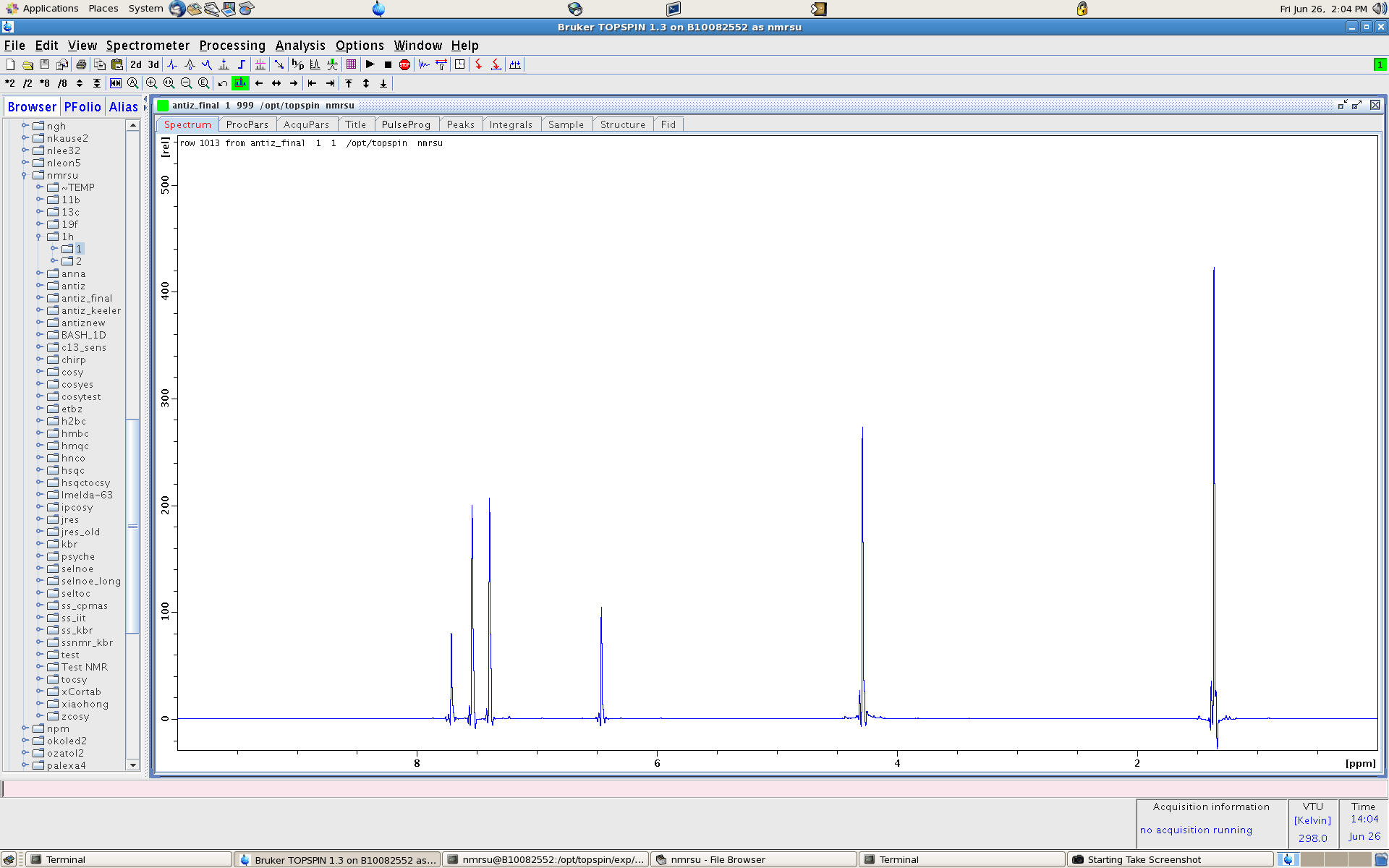
The peaks are largely J-coupling free. Just by observation we can see the integrations as 3 to 2 etc. There are some artifacts which we'll clean up
hopefully in the near future.




